Focus on medical research and serve human health
About Us
About Us
As a contract research organization (CRO) in pharmaceutical R&D and clinical studies, Panacro Pharmaceutical Technology Co., Ltd. is committed to providing overall solutions for clinical studies & IND and NDA of chemical & biological drugs and vaccines.
Since its establishment in 2004, Panacro has provided professional technical services to hundreds of customers at home and abroad in 20+ major therapeutic fields. In addition, it established offices in 20 major provinces or cities in China.
With complete and detailed standard operating procedures (SOPs), and a professional team operating in accordance with international standards (ICH-GCP), it has built close cooperation with 80% study sites in China.
Panacro focuses on providing customers with quality and efficient professional services, in order to shorten the product marketing cycle, and facilitate the product marketization.
By virtue of a professional technical team and an operation team operating in strict accordance with relevant standards, as well as professional and efficient quality service, we provide customers with highly customized solutions in a rigorous and pragmatic way based on the principle of sincere cooperation and honest operation.
- Cutting-edge medical science
- Efficient clinical operations
- Professional statistics team
- Sophisticated QC system
- Planning in vaccine
Cutting-edge medical science
Panacro has a cutting-edge medical science team dedicated to providing a full range of professional medical services for clinical studies of pharmaceutical companies at home and abroad.
With rich experience in the clinical development strategy and protocol design of anti-tumor and non-anti-tumor innovative drugs, the core executives and key team members have provided medical consulting services for many pharmaceutical companies, and have been involved in a number of successful INDs and NDAs in the world.
The core members of the Panacro medical science team, on average, have 15+ years of experience in clinical R & D in oncology, cardiovascular, surgery, anesthesia and pediatrics.
In addition, they also have years of experience in phase I-III clinical studies of chemical & biological drugs, cell therapy products, etc., for the treatment of lung cancer, liver cancer, stomach cancer, colon cancer, head and neck cancer, cervical cancer, glioma, lymphoma, multiple myeloma and leukemia in therapeutic areas of nervous system, endocrine, cardiovascular, urinary, respiratory, Infection, pain, rheumatology, dermatology, orthopedics and rare diseases.
On July 1, 2021, Panacro announced that Mr. Zhou, current professor in the Department of Biostatistics and Bioinformatics, School of Medicine at Duke University, was appointed as its Chief Consulting Scientist for the top-level design of clinical studies, application in both China and the US, international registration and global strategic layout, etc.
This will not only enable more scientific, standardized and rigorous clinical study services provided by Panacro, but also speed up the adaptive design of clinical trials and the compliance of biostatistics in China with international standards, and facilitate drug clinical studies in China to achieve leap-forward progress.
Efficient clinical operations
The clinical operation team of Panacro consists of directors, project managers and clinical monitoring team members with working experience in multinational pharmaceutical companies, dedicated to planning and organization, project management and clinical monitoring of clinical trials.
With 10+ years of experience in foreign-funded multinational pharmaceutical companies or CROs on average, and professional and comprehensive quality, the core management team members strictly implement the company's systematic, standardized and perfect SOPs and QC system, and have very rich experience in advancing progress of clinical R & D.
Panacro has established a project-centered operation management system, where the project management office is set to monitor progress, quality and operation results of projects and coordinate project resources, a project leader (PL) is designated to further decentralize evaluation and incentive authority, with project operation results and customer satisfaction to be charged by PL.
Meanwhile, a benefit-sharing mechanism based on project operation results has been established for higher vitality & quality and efficient clinical operation services for customers.
Professional statistics team
The biostatistics team at Panacro is dedicated to providing a full range of statistics-related professional services from study design to submission of study results.
Generally, the biostatistics team is usually involved from the planning stage of a clinical study, playing a crucial role in the entire project, from clinical trial design, sample size estimation to protocol writing and review.
It will develop and execute the analysis plan on time to ensure high-quality analysis results during the clinical trial.
Experienced in communicating with food and drug regulatory agencies, ethics committees and investigators, Panacro statisticians are capable of making effective communications on behalf of customers.
As a high-quality technical team with rich experience in international and domestic clinical trials, the team specializes in management of clinical trials, post-marketing and key testing items and safety data.
By strictly following NMPA data management regulations and combing with years of hands-on experience in international project operation and corresponding quality standards, the team has undertaken hundreds of clinical trial projects of various phases mainly in oncology, psychoneurology, autoimmunity, etc. and has been widely recognized by the industry.
Sophisticated QC system
With rich audit experience, it has built a comprehensive training system, and has participated in phase I-IV trials with indications involving oncology, ophthalmology, endocrinology, gynecology and neurology.
It provides all-round professional guarantee for QC throughout the projects.
According to the relevant regulations and requirements of domestic and international clinical trials, the department has established a comprehensive SOP for clinical studies, which is being continuously improved.
Professional skills training and sharing are carried out on a regular basis through the elearning system.
Through CTMS system and independently managed TMF system, the project management capability is enhanced to efficiently perform audits, correct deviations and control risks in a timely manner for the sponsor.
In accordance with relevant regulations and the requirements of Panacro’s SOPs/sponsor’s SOPs, the department conducts audits on clinical trial projects in a timely and efficient manner to ensure their cooperative operation, issues reports on problems found in the audit, and requires the respondent to take corrective and preventive actions and submit to QA for audit.
Planning in vaccine
Panacro has begun to make planning in vaccine since 2019.
As one of the initiators, we also led the establishment of the Clinical Branch of China Association for Vaccines, serving as the secretary-general unit.
By use of the expert resources and on-site resources of the Clinical Branch, Panacro has maintained close cooperation with industry experts and local disease control systems, has established a high-level vaccine clinical trial QMS, and has set up an international and professional technical team to provide quality and efficient clinical trial services for vaccine development.
In May 2020, as a part of the company’s strategic planning, Panacro’s core vaccine business was officially operated independently in the Suzhou office, mainly to improve vaccine clinical operations and build a multi-level project management model for quality services for vaccine clinical studies in China.
Panacro has facilitated 13 vaccine trials in just two years,
including several phase I/II clinical study projects of COVID-19 vaccine since the outbreak of COVID-19.
For innovative vaccines, Panacro has contributed to the country’s first recombinant vesicular project and phase I-III HPV projects.
In future, Panacro will continuously focus on providing customers with quality and efficient professional services, in order to shorten the product marketing cycle, and facilitate the product marketization.
Panacro upholds "customer-centric & striver first" core value. By virtue of a professional technical team and an operation team operating in strict accordance with relevant standards, as well as professional and efficient quality service, we provide customers with highly customized solutions in a rigorous and pragmatic way based on the principle of sincere cooperation and honest operation.
- Our Mission
- Our vision
The most reliable leading CRO in China
- Core value
Customer-centric & striver first and continuous learning, innovation & self-criticism
- Our goal
To be an Al-driven innovative CRO
Our History
Team
Management team
Chairman

In the past over one decade of development, we have always uphold the value of “customer-centric & striver first and continuous learning, innovation & self-criticism”. With the tenacity to overcome all difficulties, we have been pioneering in biologics and innovative drugs, and have built a professional team which is efficient, rigorous and pragmatic.
Taking advantage of the favorable health policy of “deepening review and approval policy and encouraging drug innovation and R & D”, Panacro based on the domestic, is dedicated to provide the fastest comprehensive service for each customer. Looking at the world, Panacro Global InnovaSolution was established in the United States to develop overseas business and start the process of internationalization.
Integrity, hard work, persistence, responsibility and gratefulness are the qualities of Panacro staff and the internal standard in our pursuit of excellence.
In the future, Panacro staff will draw upon the strengths of others, strive for progress and innovation, and build Panacro into an efficient CRO platform, thus writing a new chapter in the development of human medicine and health.
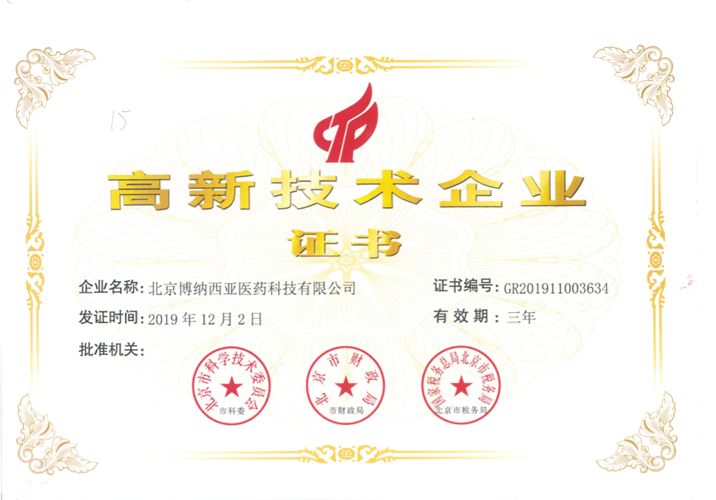
Honors
- High-tech Enterprise Certificate
- Beijing Panacro Pharmaceutical Technology Co., Ltd. Vice President Unit

- China Pharmaceutical Enterprise Development Promotion Association Vice President Unit

- High-tech Enterprise Certificate

Subsidiaries
Linzhi (Beijing) Data Technology Co., LTD
-
Biostatistics, programming and data management services
-
iDMC services
- Consisting of famous industry statistical experts, senior statisticians, and experienced EDC operation and data management teams
- Offering full services from protocol design to review Q&A/reply to CDE experts, CDISC standards, etc.

Beijing Sidafei Pharmaceutical Technology Co., LTD
- Clinical trial labor dispatch

Suzhou Panacro Pharmaceutical Technology Co., Ltd.
- Vaccine clinical trials

Panacro Global InnovaSolution (PGIS)
- Multi-Reginal clinical trial
- Cutting Edge Service

- Linzhi (Beijing) Data Technology Co., LTD
- Beijing Sidafei Pharmaceutical Technology Co., LTD
- Suzhou Panacro Pharmaceutical Technology Co., Ltd.
- Panacro Global InnovaSolution (PGIS)






